<
>

MERIDIAN M110 Fetal Monitoring System
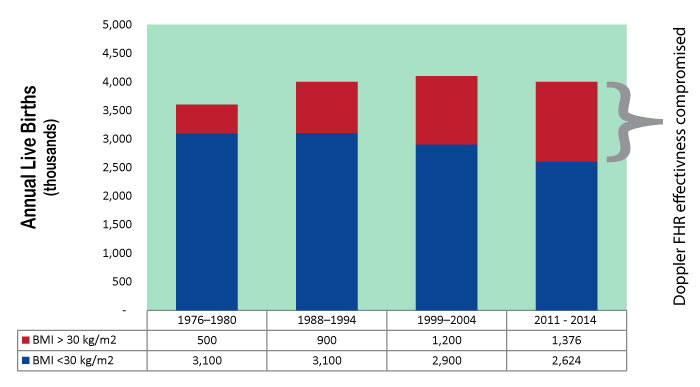
MERIDIAN's patented technology is FDA cleared to take the place of not only Doppler and tocometry, but also fetal scalp electrode and the intrauterine pressure catheter. It is no longer necessary to weigh the advantage of invasive monitoring with the risk of infection: MERIDIAN M110 provides gold-standard signal without the risk of invasive monitoring. At a time when the average BMI of obstetric patients is rising, and clinical excellence demands reliable fetal heart rate and contraction signals from all patients, MERIDIAN M110 is the only fetal monitor on the market that performs as well in obese patients as it does in lean patients. Both the fetal heart rate signal and the contraction signal deteriorate when BMI increases, compromising obstetric safety. MERIDIAN M110 solves this problem with improved technology, raising the safety profile of your unit.
View BMI Trend
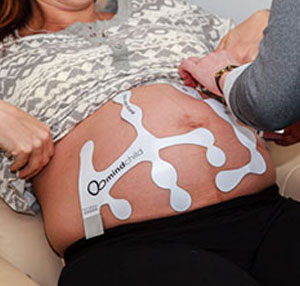
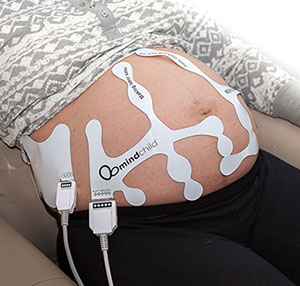
MERIDIAN M110 Disposable Electrode Patch
The MERIDIAN M110 Disposable Electrode Patch eliminates the need for skin preparation before use while providing outstanding performance and reliability across the full range of patient BMI anticipated during labor and delivery. The MERIDIAN M110 Disposable Electrode Patch replaces the fetal scalp electrode, intrauterine pressure catheter, Dopper, and TOCO sensors with a single non-invasive disposable system, while maintaining the same accuracy and sensitivity performance expected of today’s monitoring devices. Frequent repositioning associated with existing non-invasive monitoring is eliminated, as well as the risk of infection associated with invasive monitoring.
The MERIDIAN M110 Disposable Electrode Patch consists of four patches, two for the mother’s abdomen and one for each of her sides, which provides continuous fetal signal capture during fetal movement. With complete abdominal coverage, the Meridian M110 Disposable Electrode Patch delivers accurate and detailed fetal, maternal, and uterine monitoring, setting a new gold standard in fetal monitoring sensor technology.
MERIDIAN M110 Monitors the Fetal ECG
Electrocardiogram (ECG) monitoring has been widely used on adult patients for decades. In adults, signals representing a patient’s cardiac activities are collected through a set of skin surface electrodes distributed over the patient’s body.
Monitoring of fetal ECG (fECG) in this manner, however, can be difficult due to the co-existence of maternal and fetal signals acquired from a patient, as well as the relatively low fetal signal level relative to the maternal signal and other noise sources. MindChild has developed the MERIDIAN M110 Fetal Monitoring System which is a device that utilizes external electrodes and a proprietary algorithm to discern between fetal and maternal signals allowing for an external, non-invasive device to calculate the fetal heart rate.
The MERIDIAN M110 FHR Sentinel Monitoring
The MERIDIAN M110 Fetal Monitoring System overcomes a major safety hazard of traditional monitoring - the inability of Doppler technology to distinguish the maternal heart rate from the fetal heart rate when the mother and and the fetus's heart rates are similar. By tracking each signal independently, using a dedicated maternal ECG lead, MERIDIAN M110 FHR SentinelTM technology eliminates the risk.
MERIDIAN M110 Improves Quality of Interaction Between Nurse and Mother
The Meridian M110 eliminates the nursing time required to reposition the fetal monitoring transducer. The MERIDIAN M110 monitor eliminates transducer adjustment by automating sensor selection within the Meridian M110 Disposable Electrode Patch. In fact workflow analysis suggests that use of MERIDIAN M110 reduces nursing work by 1-2 hours per labor episode. This advantage is particularly pronounced when monitoring obese patients: fetal movement, maternal position, and adipose tissue conspire to make fetal monitoring a full-time job for the covering nurse. MERIDIAN M110 eliminates this hands-on work, freeing nursing staff to focus on more important aspects of patient care.
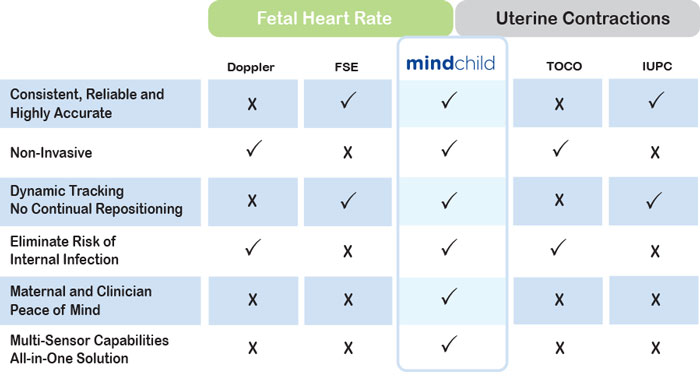
Competitive Advantages
Versus Established Technologies
Indications for Use
The MERIDIAN M110 Fetal Monitoring System is an intrapartum fetal monitor that externally measures and displays fetal heart rate (FHR), maternal heart rate (MHR), and uterine contractions (UA). The MERIDIAN M110 Fetal Monitoring System acquires and displays the FHR and UA from abdominal surface electrodes that detect the fetal ECG signals, maternal ECG signals, and of uterine muscle contraction signals. Tracings of FHR and UA are displayed onto a primary fetal monitor.
The MERIDIAN M110 Fetal Monitoring System is indicated for use on women who are at ≥ 37 completed weeks, in labor, with singleton pregnancies, using surface electrodes on the maternal abdomen. The MindChild MERIDIAN M110 is intended for use by health care professionals in a clinical setting.
© 2021-2022 Mindchild Medical, Inc. | 1600 Osgood Street | Suite 2017 | North Andover, MA 01845 | info@mindchild.com | 978.566.9880